With the approval of HUMULIN®, the first genetically modified drug, scientific community has made significant progress in developing gene edited therapies based on programmable nucleases, such as ZFNs, TALENs, EMNs and CRISPR
London
Roots Analysis has announced the addition of “Gene-Editing Beyond CRISPR: Focus on ZFN, TALENs and Meganucleases Market, 2021-2035” report to its list of offerings.
The ability of gene editing solutions to directly target and modify genomic sequences has greatly expedited the progress of gene editing therapies into clinical stages. The clinical trials data of ZFNs, TALENs and meganucleases based therapies demonstrate their potential in the treatment of various diseases, including infectious diseases and oncological disorders. In addition, several product candidates are being evaluated in discovery and preclinical stages of development for hematological disorders, genetic disorders and neurological disorders.
To order this 150+ page report, which features 100+ figures and 5+ tables, please visit
https://www.rootsanalysis.com/reports/gene-editing-market.html
Key Market Insights
Presently, close to 50 drug candidates are being evaluated across different phases of development
More than 40% of the drugs have reached clinical stage of development; of these, one drug named ZFN-758 has entered the phase II stage. This is followed by 57% drugs which are being investigated in preclinical and discovery stages.
More than 1,300 patients have been enrolled in over 28 clinical trials, worldwide
Clinical research activity, in terms of number of trials registered, is reported to have increased in the last five years. Of the total number of trials, more than 30% have already been completed, while 56% are active and still recruiting patients.
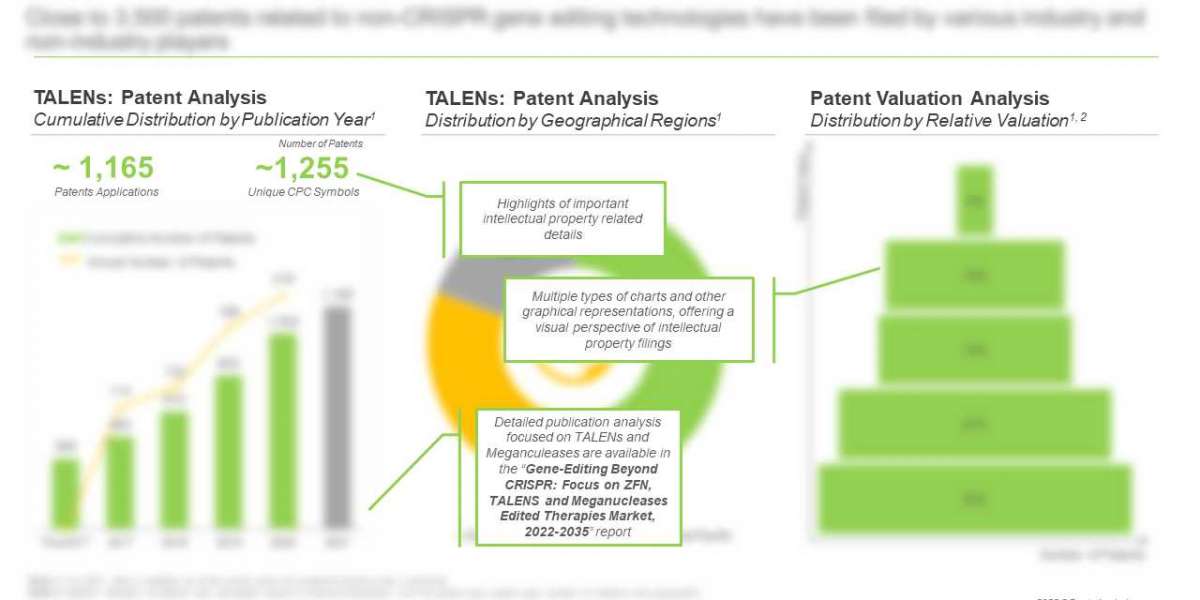
Close to 1,300 articles focused on ZFN, TALENs and Meganucleases have been published, since 2016
Owing to the rising research efforts led by several industry and non-industry players in this domain, a number or peer reviewed articles have been published in reputed scientific journals. It is worth highlighting that, in 2021 (till June) 561 articles related to TALENs were published.
Close to 20 partnerships have been inked among various industry and non-industry players
The maximum number of partnerships were established in 2021, indicating a recent rise in the interest of players involved in this domain. It is worth highlighting that, majority of the deals were licensing agreements, representing over 28% of the total number of partnerships signed followed by research and development (18%), research, development and commercialization (18%), product development and commercialization (12%), product development (12%) and clinical trial (12%) agreements.
Close to USD 1.8 billion has been invested by both private and public investors, since 2000
Of the total amount invested, over USD 884 million was raised through initial public offering (IPO), representing over 48% of the overall funding activity in this domain. Further, six instances of venture capital funding were also reported, wherein players collectively received more than USD 450 million.
The primary source of income for gene editing technology providers is anticipated to be out-licensing deals
North America is likely to capture the maximum share in the global market, followed by Europe and Asia Pacific (in terms of revenue generated from technology licensing). The market in these regions is anticipated to grow at a CAGR of 18% between 2022-2035.
To request a sample copy / brochure of this report, please visit
https://www.rootsanalysis.com/reports/gene-editing-market/request-sample.html
Key Questions Answered
- Who are the players engaged in the development of gene editing therapies beyond CRISPR?
- Which are the key drugs being developed across early and late stages of development?
- Which companies are actively involved in conducting clinical trials for ZFNs, TALENs and Meganucleases based therapies?
- What is the focus area of various publications related to ZFNs, TALENs and Meganucleases based therapies?
- What kind of partnership models are commonly adopted by industry stakeholders?
- What is the trend of capital investments in the gene editing beyond CRISPR market?
- How has the intellectual property landscape related to ZFNs, TALENs and Meganucleases based therapies evolved over the years?
- How is the current and future opportunity, related to ZFNs, TALENs and Meganucleases based therapies, likely to be distributed across key market segments?
The financial opportunity within the gene editing technologies market has been analyzed across the following key geographical regions, including:
- North America
- Europe
- Asia Pacific
The research includes detailed profiles of key players (listed below) engaged in the development of gene editing technologies; each profile features an overview of the company, its financial information (if available), details on product portfolio, recent developments, and an informed future outlook.
- Allogene Therapeutics
- bluebird bio
- Cellectis
- Cytovia Therapeutics
- Iovance Biotherapeutics
- Precision Biosciences
- Sangamo Therapeutics
For additional details, please visit
https://www.rootsanalysis.com/reports/gene-editing-market.htmlor email sales@rootsanalysis.com
You may also be interested in the following titles:
- CRISPR based Therapeutic Market by Type of Therapy, 2021-2030
- Gene Editing Services Market- Focus on CRISPR, 2019-2030
Contact:
Ben Johnson
+1 (415) 800 3415