Roots Analysis has done a detailed study on Bispecific Antibody Market (4th Edition), 2020-2030, covering key aspects of the industry’s evolution and identifying potential future growth opportunities.
To order this 370+ page report, which features 90+ figures and 110+ tables, please visit this - https://www.rootsanalysis.com/reports/view_document/bispecific-antibodies/286.html
Key Market Insights
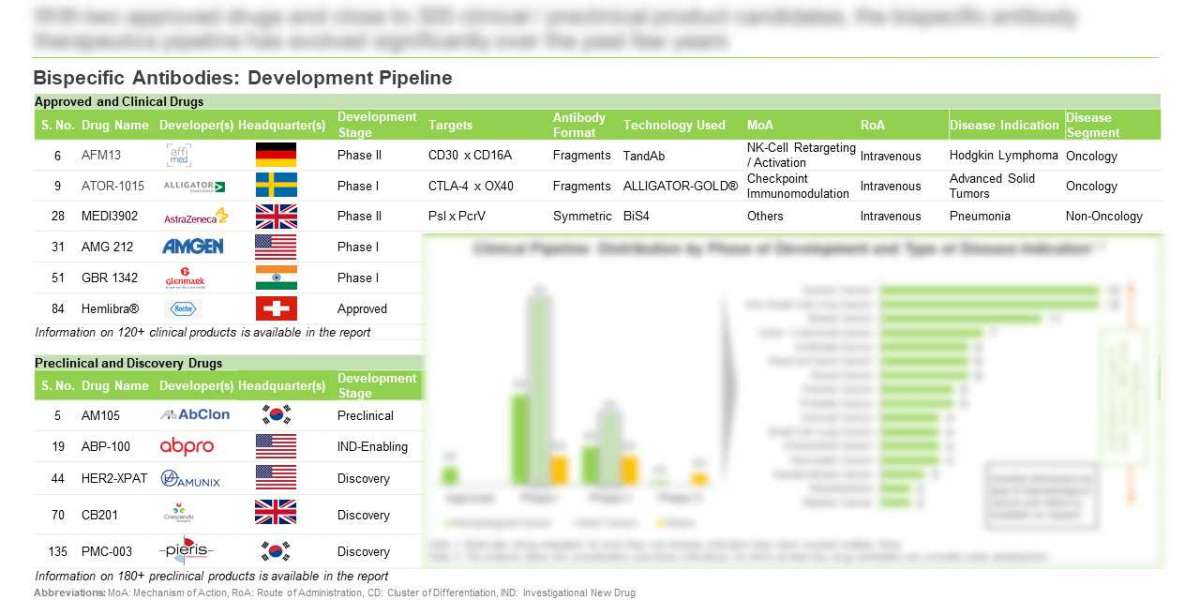
- With two approved drugs and close to 300 clinical / preclinical product candidates, the bispecific antibody therapeutics pipeline has evolved significantly over the past few years
- The pipeline features drug candidates that target a wide range of biological antigens based on different antibody formats through novel mechanisms of action; more than 50% of these act by retargeting or activation of T-Cells
- To gain a competitive edge in the market, developers are actively exploring novel biological targets and mechanisms of action to treat diverse disease indications
- Although start-ups and mid-sized firms are spearheading the innovation, several big pharmaceutical companies are also actively engaged
- In order to cater to the evolving needs of developers, technology providers have established presence across different regions; the US and EU have emerged as the key hubs
- Close to 50,000 patients were estimated to have been enrolled in clinical trials evaluating bispecific antibody therapeutics, across various geographical locations and phases of development
- The increasing interest of stakeholders in this domain can also be gauged by the rising product development / commercialization, RD and technology licensing deals being signed across various regions
- Given the complexities associated with the development of bispecific antibodies, contract organizations have become an indispensable part of the development and manufacturing process of antibody therapeutics
- In order to keep patients and healthcare professionals informed and aware of the developments, companies are deploying diverse promotional strategies for their respective products
For more information, please visit https://www.rootsanalysis.com/reports/view_document/bispecific-antibodies/286.html
Table of Contents
- PREFACE
1.1. Chapter Outlines
1.2. Research Methodology
1.3. Chapter Outlines - EXECUTIVE SUMMARY
- INTRODUCTION
3.1. Chapter Overview
3.2. Concept of an Antibody
3.3. Structure of an Antibody
3.4. Functions of an Antibody
3.5. Mechanism of Action of an Antibody
3.6. Concept of Monoclonal Antibodies
3.7. Antibody Therapeutics
3.8. Historical Evolution of Antibody Therapeutics
3.9. Types of Advanced Antibody Therapeutics
3.9.1. Fc Engineered and Glycoengineered Antibodies
3.9.2. Antibody Fragments
3.9.3. Fusion Proteins
3.9.4. Intrabodies
3.9.5. Bispecific Antibodies
3.10. Bispecific Antibody Formats
3.10.1. Single-Chain-based Formats (Fc Independent Antibody Formats)
3.10.1.1. Tandem scFvs (single-chain variable fragments) and Triple bodies
3.10.1.2. Bispecific Single-Domain Antibody Fusion Proteins
3.10.1.3. Diabodies / Diabody Derivatives
3.10.1.4. Fusion Proteins
3.10.1.5. Fusion Proteins Devoid of Fc Regions
3.10.2. Immunoglobulin G (IgG)-based Formats (Fc Dependent Antibody Formats)
3.10.2.1. Quadromas
3.10.2.2. Knobs-Into-Holes
3.10.2.3. Dual Variable Domain Ig
3.10.2.4. IgG-scFv
3.10.2.5. Two-in-one or Dual Action Fab (DAF) Antibodies
3.10.2.6. Half Molecule Exchange
3.10.2.7. κλ- Bodies
3.11. Mechanisms of Action of Bispecific Antibodies
3.11.1. Retargeting Immune Effectors (NK Cells and T Cells) to Tumor Cells
3.11.2. Directly Targeting Malignant / Tumor Cells
3.11.3. Retargeting of Toxins
3.11.5. Targeting Tumor Angiogenesis
3.11.6. Other Mechanisms
3.12. Applications of Bispecific Antibodies
4 MARKET OVERVIEW
4.1. Chapter Overview
4.2. Bispecific Antibody Therapeutics: Developer Landscape
4.2.1. Analysis by Year of Establishment
4.2.2. Analysis by Company Size
4.2.3. Analysis by Geographical Location
4.3. Bispecific Antibody Therapeutics: Clinical Pipeline
4.3.1. Analysis by Phase of Development
4.3.2. Analysis by Target Antigen
4.3.3. Analysis by Type of Antibody Format
4.3.4. Analysis by Mechanism of Action
4.3.5. Analysis by Disease Indication
4.3.6. Analysis by Therapeutic Area
4.3.7. Analysis by Broader Disease Segment
4.3.8. Analysis by Route of Administration
4.3.9. Analysis by Mode of Administration
4.3.10. Analysis by Patient Segment
4.4. Bispecific Antibody Therapeutics: Early Stage Pipeline
4.4.1. Analysis by Phase of Development
4.4.2. Analysis by Target Antigen
4.4.4. Analysis by Mechanism of Action
4.4.5. Analysis by Therapeutic Area
4.4.6. Analysis by Broader Disease Segment
4.6. Bispecific Antibody Therapeutics: Combination Therapy Candidates
4.7. Bispecific Antibody Therapeutics: Non-Industry Players
4.8. Emerging Novel Antibody Therapeutic Modalities
5 Bispecific Antibody Therapeutics: Technology Platforms
5.1. Chapter Overview
5.2. Bispecific Antibody Therapeutics: List of Technology Platforms
5.3. Bispecific Antibody Therapeutics: Technology Platform Profiles
5.3.1. Bispecific T-cell Engager (BiTE®) (Amgen)
5.3.1.1. Overview
5.3.1.2. Technology Details
5.3.1.3. Structure of BiTE® Bispecific Antibodies
5.3.1.4. Pipeline of BiTE® Bispecific Antibodies
5.3.1.5. Advantages of BiTE® Bispecific Antibodies
5.3.1.6. Partnerships and Collaborations
5.3.2. DuoBody® (Genmab)
5.3.2.1. Overview
5.3.2.2. Technology Details
5.3.2.3. Structure of DuoBody® Bispecific Antibodies
5.3.2.4. Pipeline of DuoBody® Bispecific Antibodies
5.3.2.5. Advantages of DuoBody® Bispecific Antibodies
5.3.2.6. Partnerships and Collaborations
5.3.3. Xmab™ Antibody Engineering Platform (Xencor)
5.3.3.1. Overview
5.3.3.2. Technology Details
5.3.3.3. Pipeline of Xmab™ Bispecific Antibodies
5.3.3.4. Advantages of Xmab™ Bispecific Antibodies
5.3.3.5. Partnerships and Collaborations
5.3.4. WuXibodyTM Bispecific Engineering Platform (WuXi Biologics)
5.3.4.1. Overview
5.3.4.2. Pipeline of WuXibodyTM Bispecific Antibodies
5.3.4.3. Advantages of WuXibodyTM Bispecific Antibodies
5.3.4.4. Partnerships and Collaborations
5.3.5. Anticalin® (Pieris Pharmaceuticals)
5.3.5.1. Overview
5.3.5.2. Structure of Anticalin® Bispecific Fusion Proteins
5.3.5.3. Pipeline of Anticalin® Bispecific Fusion Proteins
5.3.5.4. Advantages of Anticalin® Bispecific antibody Platform
5.3.5.5. Partnerships and Collaborations
5.3.6. Azymetric™ (Zymeworks)
5.3.6.1. Overview
5.3.6.2. Technology Details
5.3.6.3. Structure of Azymetric™ Bispecific Antibodies
5.3.6.4. Pipeline of Azymetric™ Bispecific Antibodies
5.3.6.5. Advantages of the AzymetricTM Bispecific Antibodies
5.3.6.6. Partnerships and Collaborations
5.4. Geographical Distribution of Technology Providers
5.5. Bispecific Antibody Technology Platforms: Comparative Analysis
6 DRUG PROFILES
6.1. Chapter Overview
6.2. Marketed Drug Profiles
6.2.1. Blincyto™ / Blinatumomab / AMG103 / MT103 (Amgen)
6.2.1.1. Company Overview
6.2.1.1.1. Financial Performance
6.2.1.2. Drug Overview
6.2.1.2.1. Mechanism of Action and Targets
6.2.1.2.2. Dosage
6.2.1.2.3. Current Development Status
6.2.1.2.4. Development Process
6.2.1.2.5. Annual Sales
6.2.2. Hemlibra® / Emicizumab / RG6013 / ACE910 / RO5534262 (Chugai Pharmaceutical / Roche)
6.2.2.1. Company Overview
6.2.2.1.1. Financial Performance
6.2.2.2. Drug Overview
6.2.2.2.1. Mechanism of Action and Targets
6.2.2.2.2. Dosage
6.2.2.2.4. Development Process
6.2.2.2.5. Annual Sales
6.3. Late Stage Drug Profiles
6.4. RG7716 / RO6867461 / Faricimab (Roche / Genentech)
6.4.1 Drug Overview
6.5. Ozoralizumab / TS-152 / ATN103 (Ablynx / Eddingpharm / Taisho Pharmaceuticals)
6.5.1 Drug Overview
6.6. ABT-165 (AbbVie)
6.6.1 Overview of Drug, Current Development Status and Clinical Results
6.7. ABY-035 (Affibody)
6.7.1. Drug Overview
6.8. AFM13 (Affimed)
6.8.1 Drug Overview
6.9. AMG 570 / MEDI0700 (Amgen)
6.9.1. Drug Overview
6.10. KN026 (Alphamab)
6.10.1. Drug Overview
6.11. KN046 (Alphamab)
6.11.1. Drug Overview
6.12. M1095 / ALX-0761 (Merck / Ablynx / Avillion)
6.12.1. Drug Overview
6.13. M7824 / Bintrafusp Alfa (Merck / GlaxoSmithKline)
6.13.1. Drug Overview
6.14. MCLA-128 (Merus)
6.14.1. Drug Overview
6.15. MEDI3902 / Gremubamab (MedImmune /AstraZeneca)
6.15.1. Drug Overview
6.16. Drug Overview
6.17. REGN1979 (Regeneron)
6.17.1. Drug Overview 6.18. ZW25 (Zymeworks)
6.18.1. Drug Overview
7 KEY INSIGHTS
7.1. Chapter Overview
7.2. Bispecific Antibody Therapeutics: Analysis by Therapeutic Area and Phase of Development
7.3. Bispecific Antibody Therapeutics: Spider-Web Analysis based on Mechanism of Action
7.4. Bispecific Antibody Therapeutics: Two-Dimensional Scatter Plot Analysis based on Target Combinations
7.4.1 Key Parameters
7.5. Logo Landscape: Analysis of Developers by Company Size
8 BENCHMARK ANALYSIS: BIG PHARMA PLAYERS
8.1. Chapter Overview
8.2. Top Pharmaceutical Companies
8.2.1. Analysis by Target Antigen
8.2.2. Analysis by Type of Antibody Format
8.2.3. Analysis by Mechanism of Action
8.2.4. Analysis by Therapeutic Area
8.2.5. Analysis by Type of Partnership
9 PARTNERSHIPS AND COLLABORATIONS
9.1. Chapter Overview
9.2. Partnership Models
9.3. Bispecific Antibody Therapeutics: Partnerships and Collaborations
9.3.1. Analysis by Year of Partnership
9.3.2. Analysis by Type of Partnership
9.3.3. Analysis by Therapeutic Area
9.3.4. Most Active Developers: Analysis by Number of Partnerships
9.3.5. Most Active Contract Manufacturers: Analysis by Number of Manufacturing Agreements
9.3.6. Regional Analysis
9.3.7. Intercontinental and Intracontinental Agreements
10 CONTRACT SERVICES FOR BISPECIFIC ANTIBODY THERAPEUTICS
10.1. Chapter Overview
10.2. Manufacturing of Bispecific Antibody Therapeutics
10.2.1. Key Manufacturing Considerations and Challenges
10.2.2. Contract Manufacturing Organizations (CMOs)
10.2.2.1. Introduction to CMOs
10.2.2.2. Bispecific Antibody Therapeutics: List of CMOs
10.2.3. Contract Research Organizations (CROs)
10.2.3.1. Introduction to CROs
10.2.3.2. Bispecific Antibody Therapeutics: List of CROs
10.3. Key Considerations for Selecting a Suitable CMO / CRO Partner
11 CLINICAL TRIAL ANALYSIS
11.1. Chapter Overview
11.2. Methodology
11.3. Bispecific Antibody Therapeutics: Clinical Trial Analysis
11.3.1. Analysis by Trial Registration Year
11.3.2. Analysis by Trial Recruitment Status
11.3.3. Analysis by Trial Phase
11.3.4. Analysis by Trial Design
11.3.5. Analysis by Disease Indication
11.3.6. Analysis by Therapeutic Area
11.3.7. Most Active Players
11.3.8. Analysis by Number of Clinical Trials and Geography
11.3.9. Analysis by Enrolled Patient Population and Geography
12 CASE STUDY: REGULATORY GUIDELINES FOR BISPECIFIC ANTIBODIES
12.1. Chapter Overview
12.2. Guidelines Issued by Regulatory Authorities
12.2.1. US Food and Drug Administration (FDA)
12.2.1.1. Pharma Companies Response to the FDA Draft Guidance
12.2.2. World Health Organization (WHO)
12.2.3. The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use
13 CASE STUDY: PROMOTIONAL / MARKETING STRATEGIES
13.1. Chapter Overview
13.2. Overview of Channels Used for Promotional Campaigns
13.3. Summary: Product Website Analysis
13.4. Summary: Patient Support Services and Informative Downloads
13.5. Promotional Analysis: Blincyto™
13.5.1. Drug Overview
13.5.2. Product Website analysis
13.5.2.1. Messages for Healthcare Professionals
13.5.2.1.1. For MRD Positive B‑cell precursor ALL
13.5.2.1.2. For Relapsed or Refractory B-cell precursor ALL
13.5.2.2. Message for Patients
13.5.3. Patient Support Services and Informative Downloads
13.5.4. Other Promotional Strategies
13.5.4.1. Presence in Conferences
13.6. Promotional Analysis: Hemlibra®
13.6.1. Drug Overview
13.6.2. Product Website Analysis
13.6.2.1. Messages for Healthcare Professionals
13.6.2.1.1. For Hemophilia A without Factor VIII Inhibitors
13.6.2.1.2. For Hemophilia A with Factor VIII Inhibitors
13.6.2.3. Messages for Patients
13.6.3. Patient Support Services and Informative Downloads
13.6.3.1. Co-pay Program
13.6.3.2. Independent Co-pay Assistance Foundation
13.6.3.3. Genentech Patient Foundation
13.6.4. Other Promotional Strategies
13.6.4.1. Presence in Conferences
14 SWOT ANALYSIS
14.1. Chapter Overview
14.2. Strengths
14.3. Weaknesses
14.4. Opportunities
14.5. Threats
14.6. Concluding Remarks
15 MARKET FORECAST AND OPPORTUNITY ANALYSIS
15.1. Chapter Overview
15.2. Scope and Limitations
15.3. Forecast Methodology and Key Assumptions
15.4. Overall Bispecific Antibody Therapeutics Market, 2019-2030
15.4.1. Bispecific Antibody Therapeutics Market: Analysis by Therapeutic Area
15.4.2. Bispecific Antibody Therapeutics Market: Analysis by Mechanism of Action
15.4.3. Bispecific Antibody Therapeutics Market: Analysis by Target Antigen
15.4.4. Bispecific Antibody Therapeutics Market: Analysis by Antibody Format
15.4.5. Bispecific Antibody Therapeutics Market: Analysis by Key Players
15.4.6. Bispecific Antibody Therapeutics Market: Analysis by Geography
15.5. Bispecific Antibody Market: Value Creation Analysis
15.6. Bispecific Antibody Therapeutics Market: Product-wise Sales Forecasts
15.6.1. Blincyto
15.6.1.1. Target Patient Population
15.6.1.2. Sales Forecast
15.6.1.3. Net Present Value
15.6.1.4. Value Creation Analysis
15.6.2. Hemlibra
15.6.2.1. Target Patient Population
15.6.2.2. Sales Forecast
15.6.2.3. Net Present Value
15.6.2.4. Value Creation Analysis
15.6.3. RG7716
15.6.3.1. Target patient Population
15.6.3.2. Sales Forecast
15.6.3.3. Net Present Value
15.6.3.4. Value Creation Analysis
15.6.4. Ozoralizumab
15.6.4.1. Target Patient Population
15.6.4.2. Sales Forecast
15.6.4.3. Net Present Value
15.6.4.4. Value Creation Analysis
15.6.5. ABY-035
15.6.5.1. Target Patient Population
15.6.5.2. Sales Forecast
15.6.5.3. Net Present Value
15.6.5.4. Value Creation Analysis
15.6.6. AFM13
15.6.6.1. Target Patient Population
15.6.6.2. Sales Forecast
15.6.6.3. Net Present Value
15.6.6.4. Value Creation Analysis
15.6.7. M1095
15.6.7.1. Target Patient Population
15.6.7.2. Sales Forecast
15.6.7.3. Net Present Value
15.6.7.4. Value Creation Analysis
15.6.8. MEDI3902
15.6.8.1. Target Patient Population
15.6.8.2. Sales Forecast
15.6.8.3. Net Present Value
15.6.8.4. Value Creation Analysis
15.6.9. ABT-981
15.6.9.1. Target Patient Population
15.6.9.2. Sales Forecast
15.6.9.3. Net Present Value
15.6.9.4. Value Creation Analysis
15.6.10. SAR156597
15.6.10.1. Target Patient Population
15.6.10.2. Sales Forecast
15.6.10.3. Net Present Value
15.6.10.4. Value Creation Analysis
16 CONCLUDING REMARKS
17 EXECUTIVE INSIGHTS
17.1. Chapter Overview
17.2. CytomX Therapeutics
17.2.1. Company Snapshot
17.2.2. Interview Transcript: Siobhan Pomeroy, Senior Director, Business Development (Q3 2017)
17.3. F-star
17.3.1. Company Snapshot
17.3.2. Interview Transcript: Jane Dancer, Chief Business Officer (Q3 2017)
17.4. Innovent Biologics
17.4.1. Company Snapshot
17.4.2. Interview Transcript: Yinjue Wang, Associate Director, Process Development (Q3 2017)
17.5. Synimmune
17.4.1. Company Snapshot
17.4.2. Interview Transcript: Ludger Große-Hovest, Chief Scientific Officer, and Martin Steiner, Chief Executive Officer
- APPENDIX 1: TABULATED DATA
- APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
For more information please click on the following link:
https://www.rootsanalysis.com/reports/view_document/bispecific-antibodies/286.html
Other Recent Offerings
- Global Preventive Vaccines Market, 2020-2030
- Endocannabinoid System Targeted Therapeutics Market, 2019-2030
- Antibody Contract Manufacturing Market, 2020-2030
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com
Contact Information
Roots Analysis Private Limited
Ben Johnson
+1 (415) 800 3415
Facebook - https://www.facebook.com/RootsAnalysis
LinkedIn - https://www.linkedin.com/company/roots-analysis/mycompany/
Twitter - https://twitter.com/RootsAnalysis
Medium - https://medium.com/@RootsAnalysis
Pinterest - https://in.pinterest.com/RootsanalysisPin/_saved/