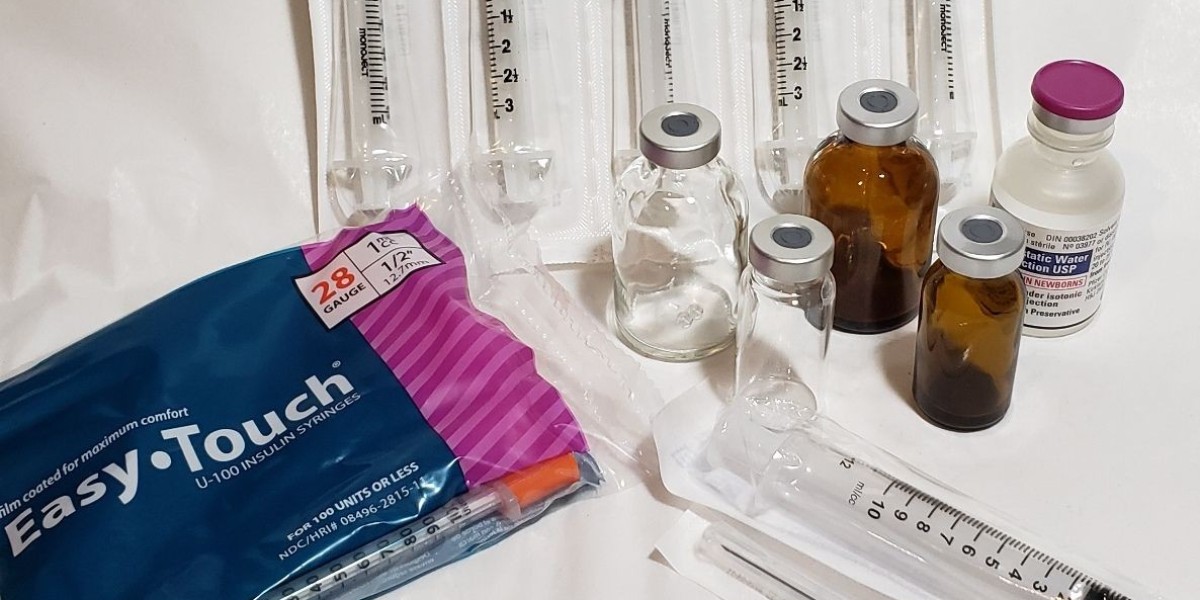
HCG supplies play a significant role in many medical and laboratory environments, where proper handling and quality control are essential. These materials may include vials, diluents, syringes, packaging components, and storage accessories associated with HCG preparation and distribution. Since they involve biological reconstitution solution and pharmaceutical materials, the management of HCG supplies requires consistent awareness of safety standards, documentation, and regulatory compliance. Understanding how these supplies move through the supply chain—from manufacturing to storage and eventual clinical use—helps ensure they remain safe, sterile, and reliable throughout their lifecycle.
A critical element of HCG supply management is maintaining proper storage conditions. HCG products tend to be sensitive to temperature changes, meaning they have to be kept within specific ranges to preserve stability. Many facilities rely on temperature-controlled environments, monitoring systems, and insulated packaging to keep up integrity during storage or transportation. If storage guidelines are not followed, the material may lose its effectiveness or become unsuitable for clinical distribution. Because of this, healthcare facilities typically implement strict procedures for receiving, inspecting, and organizing HCG supplies the moment shipments arrive.
Regulatory oversight plays a major role in ensuring that HCG supplies meet established safety and quality standards. Manufacturers are required to check out pharmaceutical production guidelines, maintain sterile environments, and document each batch with clear labeling and lot numbers. Distributors must follow transportation regulations, while pharmacies and healthcare providers must follow national and regional laws governing how HCG supplies are stored, tracked, and documented. These regulations help avoid the circulation of counterfeit or improperly handled materials and protect both healthcare professionals and patients from potential risks.
Certainly one of the most important aspects of managing HCG supplies is quality assurance and traceability. Healthcare facilities often rely on inventory systems that track expiration dates, batch numbers, and storage history to ensure each item meets safety requirements. Monitoring systems may include barcode scanning, digital logs, and automated alerts for materials nearing expiration. Proper documentation ensures that supplies could be traced back through the supply chain if issues arise, helping maintain accountability and transparency. This systematic approach also reduces the likelihood of errors during storage or preparation.
Overall, the handling and oversight of HCG supplies demonstrate how important safety protocols, accurate record-keeping, and regulatory compliance are within medical and laboratory environments. These supplies require attention to storage conditions, manufacturing standards, and documentation processes to maintain integrity from the moment they are produced until they reach the facility that will use them. As technology advances, digital tracking tools, improved packaging, and more robust regulatory frameworks continue steadily to strengthen quality control in pharmaceutical supply management. By understanding these processes, organizations can better make sure that their HCG supplies remain safe, reliable, and compliant with industry standards.