Roots Analysis has done a detailed study on Oligonucleotide Synthesis Market : Focus on Research, Diagnostic and Therapeutic Applications, 2020-2030.”” covering key aspects of the industry’s evolution and identifying potential future growth opportunities.
To order this 300+ page report, which features 140+ figures and 190+ tables, please visit this - https://www.rootsanalysis.com/reports/view_document/oligonucleotide-synthesis/304.html
Key Market Insights
- More than 80 companies currently claim to offer manufacturing related services, at different scales of operations, for a variety of oligonucleotide-based products
- A wide range of services related to the synthesis, modification and purification of oligonucleotides, are currently offered within a fragmented service provider landscape that is mostly concentrated in the developed geographies
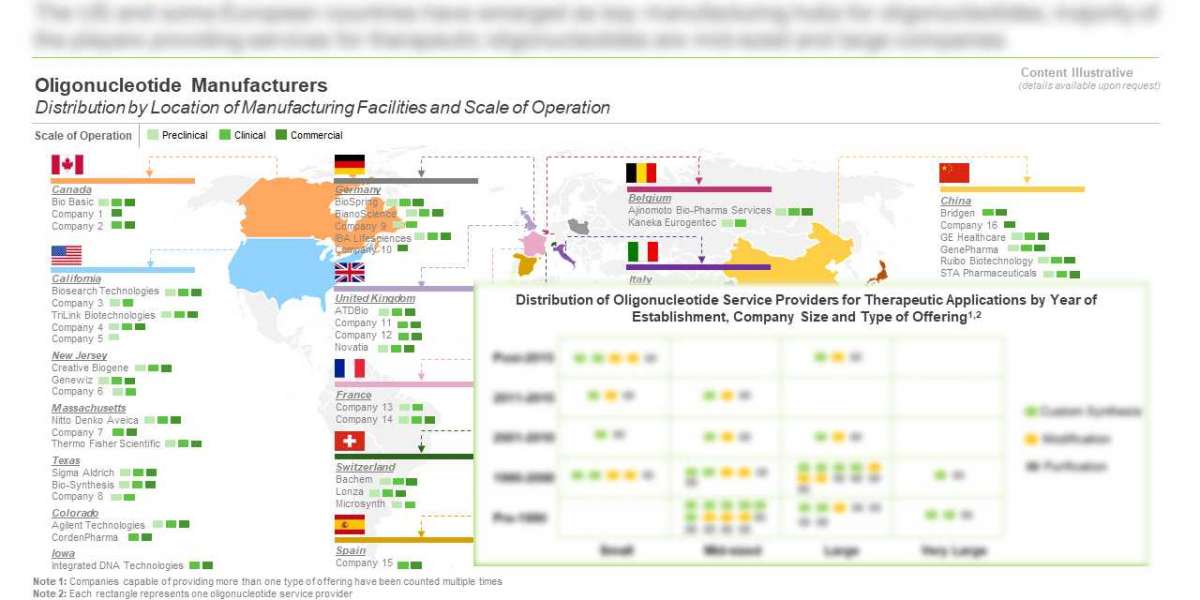
- In order to cater to the growing needs of clients / sponsors, companies have established presence across different regions; the US and some European nations have emerged as current hubs for oligonucleotide production
- Players involved in this domain are steadily expanding their capabilities in order to enhance their respective service portfolios and thereby, achieve an edge over competing firms
- Service providers are actively investing in expansion projects to upgrade existing capabilities and capacity; several partnerships, mostly focused on offering manufacturing and supply services, have been forged
- Over the past few years, more than 270 trials of oligonucleotide-based interventions, across various phases of development and to treat a diverse range of diseases, have been registered across different centers worldwide
- Most of the global, annual oligonucleotide manufacturing capacity belongs to established service providers, accounting for over 80% of available capacity across various geographies
- The demand for manufacturing of oligonucleotide-based products is expected to increase in the coming years; we believe that the stakeholders may have to expand their respective capacities to ensure consistent supply
- We expect oligonucleotide-based drug developers to continue to outsource their manufacturing operations in the short to mid-term; service-based revenues are estimated to grow at an annualized rate of more than 10%
- In the long-term, the projected opportunity is anticipated to be well distributed across various therapeutic areas, scales of operation and across companies of different sizes
- In order to account for the uncertainties associated with the Oligonucleotide Synthesis Market Growth and to add robustness to our model, we have provided three forecast scenarios, portraying the conservative, base and optimistic tracks of the market’s evolution.
For more information, please visit https://www.rootsanalysis.com/reports/view_document/oligonucleotide-synthesis/304.html
Table of Contents
- PREFACE
1.1. Scope of the Report
1.2. Research Methodology
1.3. Chapter Outlines
EXECUTIVE SUMMARY
- INTRODUCTION
3.1. Context and Background
3.2. Overview of Oligonucleotide-based Products
3.3. Types of Oligonucleotides
3.3.1. Antisense Oligonucleotides
3.3.2. Aptamers
3.3.3. miRNA
3.3.4. shRNA
3.3.5. siRNA
3.3.6. Other Oligonucleotides
3.4. Custom Synthesis of Oligonucleotides
3.4.1. Process Development and Characterization
3.4.2. Analytical Method Development
3.4.3. Method Validation and Testing
3.4.4. Quality Control and Quality Assurance
3.4.5. Challenges Associated with Custom Synthesis of Oligonucleotides
3.5. Chemical Modification of Oligonucleotides
3.5.1. Backbone Modification
3.5.2. Sugar Ring Modification
3.6. Purification of Oligonucleotides
3.6.1. Desalting
3.6.2. Cartridge Purification
3.6.3. Polyacrylamide Gel Electrophoresis (PAGE)
3.6.4. High Performance Liquid Chromatography (HPLC)
3.7. Outsourcing Oligonucleotide Manufacturing
3.7.1. Need for Outsourcing
3.7.2. Commonly Outsourced Operations
3.7.3. Advantages of Outsourcing Manufacturing Operations
3.7.4. Guidelines for Selecting a Service Provider
3.8. Growth Drivers and Roadblocks to Oligonucleotide Manufacturing
3.9. Recent Developments and Upcoming Trends
- MARKET LANDSCAPE: OLIGONUCLEOTIDE MANUFACTURERES (RESEARCH AND DIAGNOSTIC APPLICATIONS)
4.1. Chapter Overview
4.2. Oligonucleotide Manufacturers Focused on Research and Diagnostic Applications: Overall Market Landscape
4.2.1. Analysis by Year of Establishment
4.2.2. Analysis by Company Size
4.2.3. Analysis by Scale of Operation
4.2.4. Analysis by Geographical Location
4.2.5. Analysis by Location of Manufacturing Facilities
4.2.6. Analysis by Regulatory Accreditations / Certifications
4.2.7. Analysis by Type of Oligonucleotide Manufactured
4.2.8. Analysis by Type of Offering
4.2.9. Analysis by Type of Manufacturing Service(s) Offered
4.2.10. Analysis by Type of Modification(s) Offered
4.2.11. Analysis by Type of Purification Method(s) Used
4.2.12. Analysis by Compliance to cGMP Standards
- MARKET LANDSCAPE: OLIGONUCLEOTIDE MANUFACTURERES (THERAPEUTIC APPLICATIONS)
5.1. Chapter Overview
5.2. Oligonucleotide Manufacturers Focused on Therapeutic Applications: Overall Market Landscape
5.2.1. Analysis by Year of Establishment
5.2.2. Analysis by Company Size
5.2.3. Analysis by Scale of Operation
5.2.4. Analysis by Geographical Location
5.2.5. Analysis by Location of Manufacturing Facilities
5.2.6. Analysis by Regulatory Accreditations / Certifications
5.2.7. Analysis by Type of Oligonucleotide Manufactured
5.2.8. Analysis by Type of Offering
5.2.9. Analysis by Type of Manufacturing Service(s) Offered
5.2.10. Analysis by Type of Modification(s) Offered
5.2.11. Analysis by Type of Purification Method(s) Used
5.2.12. Analysis by Compliance to cGMP Standards
- COMPANY COMPETITIVENESS ANALYSIS: OLIGONUCLEOTIDE MANUFACTURES (RESEARCH AND DIAGNOSTIC APPLICATIONS)
6.1. Chapter Overview
6.2. Key Assumptions and Methodology
6.3. Company Competitiveness Analysis: Oligonucleotide Manufacturers Focused on Research and Diagnostic Applications
6.3.1. Oligonucleotide Manufacturers in North America
6.3.2. Oligonucleotide Manufacturers in Europe
6.3.3. Oligonucleotide Manufacturers in Asia-Pacific
- COMPANY COMPETITIVENESS ANALYSIS: OLIGONUCLEOTIDE MANUFACTURERS (THERAPEUTIC APPLICATIONS)
7.1. Chapter Overview
7.2. Key Assumptions and Methodology
7.3. Company Competitiveness Analysis: Oligonucleotide Manufacturers Focused on Therapeutic Applications
7.3.1. Oligonucleotide Manufacturers in North America
7.3.2. Oligonucleotide Manufacturers in Europe
7.3.3. Oligonucleotide Manufacturers in Asia-Pacific
- COMPANY PROFILES: OLIGONUCLEOTIDE MANUFACTURERS (RESEARCH AND DIAGNOSTIC APPLICATIONS)
8.1. Chapter Overview
8.2. Ajinomoto Bio-Pharma Services
8.2.1. Company Overview
8.2.2. Service Portfolio
8.2.3. Manufacturing Facilities and Capabilities
8.2.4. Recent Developments and Future Outlook
8.3. Integrated DNA Technologies
8.3.1. Company Overview
8.3.2. Service Portfolio
8.3.3. Manufacturing Facilities and Capabilities
8.3.4. Recent Developments and Future Outlook
8.4. Kaneka Eurogentec
8.4.1. Company Overview
8.4.2. Service Portfolio
8.4.3. Manufacturing Facilities and Capabilities
8.4.4. Recent Developments and Future Outlook
8.5. LGC Biosearch Technologies
8.5.1. Company Overview
8.5.2. Service Portfolio
8.5.3. Manufacturing Facilities and Capabilities
8.5.4. Recent Developments and Future Outlook
8.6. Microsynth
8.6.1. Company Overview
8.6.2. Service Portfolio
8.6.3. Manufacturing Facilities and Capabilities
8.6.4. Recent Developments and Future Outlook
8.7. Sigma Aldrich
8.7.1. Company Overview
8.7.2. Financial Information
8.7.3. Service Portfolio
8.7.4. Manufacturing Facilities and Capabilities
8.7.5. Recent Developments and Future Outlook
8.8. Thermo Fisher Scientific
8.8.1. Company Overview
8.8.2. Financial Information
8.8.3. Service Portfolio
8.8.4. Manufacturing Facilities and Capabilities
8.8.5. Recent Developments and Future Outlook
- COMPANY PROFILES: OLIGONUCLEOTIDE MANUFACTURERS (THERAPEUTIC APPLICATIONS)
9.1. Chapter Overview
9.2. Agilent Technologies
9.2.1. Company Overview
9.2.2. Financial Information
9.2.3. Service Portfolio
9.2.4. Manufacturing Facilities and Capabilities
9.2.5. Recent Developments and Future Outlook
9.3. BioSpring
9.3.1. Company Overview
9.3.2. Service Portfolio
9.3.3. Manufacturing Facilities and Capabilities
9.3.4. Recent Developments and Future Outlook
9.4. CordenPharma
9.4.1. Company Overview
9.4.2. Service Portfolio
9.4.3. Manufacturing Facilities and Capabilities
9.4.4. Recent Developments and Future Outlook
9.5. Nitto Denko Avecia
9.5.1. Company Overview
9.5.2. Service Portfolio
9.5.3. Manufacturing Facilities and Capabilities
9.5.4. Recent Developments and Future Outlook
9.6. TriLink Biotechnologies
9.6.1. Company Overview
9.6.2. Service Portfolio
9.6.3. Manufacturing Facilities and Capabilities
9.6.4. Recent Developments and Future Outlook
- PARTNERSHIPS AND COLLABORATIONS
10.1. Chapter Overview
10.2. Partnership Models
10.3. Oligonucleotide Manufacturers: Recent Partnerships and Collaborations
10.3.1. Analysis by Year of Partnership
10.3.2. Analysis by Type of Partnership
10.3.3. Analysis by Type of Partner
10.3.4. Most Active Players: Analysis by Number of Partnerships
10.3.5. Geographical Analysis
10.3.5.1. Most Active Players: Geographical Distribution by Number of Partnerships
10.3.5.2. Intercontinental and Intracontinental Agreements
- RECENT EXPANSIONS
11.1. Chapter Overview
11.2. Oligonucleotide Manufacturers: Recent Expansions
11.2.1. Analysis by Year of Expansion
11.2.2. Analysis by Type of Expansion
11.2.3. Analysis by Application
11.2.4. Analysis by Location of Facility
11.2.5. Analysis by Expanded Facility Area
11.2.6. Analysis by Expanded Scale of Operation
11.3.7. Most Active Players: Analysis by Number of Expansions
11.3.8. Geographical Analysis
11.3.8.1. Continent-wise Distribution
11.3.8.2. Country-wise Distribution
- CLINICAL TRIAL ANALYSIS
12.1. Chapter Overview
12.2. Scope and Methodology
12.3. Clinical Trial Analysis: Oligonucleotide-based Drug Products
12.3.1. Analysis by Trial Registration Year
12.3.2. Analysis by Phase of Development
12.3.3. Analysis by Type of Oligonucleotide
12.3.4. Analysis by Type of Oligonucleotide and Phase of Development
12.3.5. Analysis by Trial Recruitment Status
12.3.6. Analysis by Trial Focus Area
12.3.7. Analysis by Target Therapeutic Area
12.3.8. Geographical Analysis by Number of Clinical Trials
12.3.9. Geographical Analysis by Enrolled Patient Population
12.3.10. Analysis by Type of Sponsor / Collaborator
12.3.11. Most Active Players: Analysis by Number of Registered Trials
- CAPACITY ANALYSIS
13.1. Chapter Overview
13.2. Key Assumptions and Methodology
13.3. Oligonucleotide Manufacturers: Global, Annual Capacity
13.3.1. Analysis by Size of Manufacturer
13.3.2. Analysis by Scale of Operation
13.3.3. Analysis by Location of Manufacturing Facility
- DEMAND ANALYSIS
14.1. Chapter Overview
14.2. Key Assumptions and Methodology
14.3. Global Demand for Oligonucleotide Manufacturing
14.3.1. Global Commercial Demand for Oligonucleotide Manufacturing
14.3.1.1. Analysis by Type of Oligonucleotide
14.3.1.2. Analysis by Target Therapeutic Area
14.3.1.3. Analysis by Geography
14.3.2. Global Clinical Demand for Oligonucleotide Manufacturing
14.3.2.1. Analysis by Type of Oligonucleotide
14.3.2.2. Analysis by Phase of Development
14.3.2.3. Analysis by Target Therapeutic Area
14.3.2.4. Analysis by Geography
14.4. Demand and Supply Analysis
14.4.1. Demand and Supply Analysis (Scenario 1)
14.4.2. Demand and Supply Analysis (Scenario 2)
14.4.3. Demand and Supply Analysis (Scenario 3)
- MARKET SIZING AND OPPORTUNITY ANALYSIS
15.1. Chapter Overview
15.2. Key Assumptions and Forecast Methodology
15.3. Overall Oligonucleotide Manufacturing Market, 2020-2030
15.4. Oligonucleotide Manufacturing Market, 2020-2030: Analysis by Type of Manufacturing
15.4.1. Custom Oligonucleotide Manufacturing Market, 2020-2030
15.4.2. Large-scale Oligonucleotide Manufacturing Market, 2020-2030
15.4.2.1 Oligonucleotide Manufacturing Market, 2020-2030: Analysis by Type of Oligonucleotide Manufactured
15.4.2.1.1. Oligonucleotide Manufacturing Market for Antisense Oligonucleotides, 2020-2030
15.4.2.1.2. Oligonucleotide Manufacturing Market for miRNA, 2020-2030
15.4.2.1.3. Oligonucleotide Manufacturing Market for shRNA, 2020-2030
15.4.2.1.4. Oligonucleotide Manufacturing Market for siRNA, 2020-2030
15.4.2.1.5. Oligonucleotide Manufacturing Market for Other Oligonucleotides, 2020-2030
15.4.2.2. Oligonucleotide Manufacturing Market, 2020-2030: Analysis by Scale of Operation
15.4.2.2.1. Oligonucleotide Manufacturing Market for Clinical Scale Operations, 2020-2030
15.4.2.2.2. Oligonucleotide Manufacturing Market for Commercial Scale Operations, 2020-2030
15.4.2.3. Oligonucleotide Manufacturing Market, 2020-2030: Analysis by Purpose of Production
15.4.2.3.1. Oligonucleotide Manufacturing Market for In-House Operations, 2020-2030
15.4.2.3.2. Oligonucleotide Manufacturing Market for Outsourced Operations, 2020-2030
15.4.2.4. Oligonucleotide Manufacturing Market, 2020-2030: Analysis by Target Therapeutic Area
15.4.2.4.1. Oligonucleotide Manufacturing Market for Autoimmune Disorders, 2020-2030
15.4.2.4.2. Oligonucleotide Manufacturing Market for Cardiovascular Disorders, 2020-2030
15.4.2.4.3. Oligonucleotide Manufacturing Market for Genetic Disorders, 2020-2030
15.4.2.4.4. Oligonucleotide Manufacturing Market for Infectious Diseases, 2020-2030
15.4.2.4.5. Oligonucleotide Manufacturing Market for Metabolic Disorders, 2020-2030
15.4.2.4.6. Oligonucleotide Manufacturing Market for Neuromuscular Disorders, 2020-2030
15.4.2.4.7. Oligonucleotide Manufacturing Market for Oncological Disorders, 2020-2030
15.4.2.4.8. Oligonucleotide Manufacturing Market for Ophthalmic Disorders, 2020-2030
15.4.2.4.9. Oligonucleotide Manufacturing Market for Other Therapeutic Areas, 2020-2030
15.4.2.5. Oligonucleotide Manufacturing Market, 2020-2030: Analysis by Size of Manufacturer
15.4.2.5.1. Oligonucleotide Manufacturing Market for Small Companies, 2020-2030
15.4.2.5.2. Oligonucleotide Manufacturing Market for Mid-sized Companies, 2020-2030
15.4.2.5.3. Oligonucleotide Manufacturing Market for Large Companies, 2020-2030
15.4.2.6. Oligonucleotide Manufacturing Market, 2020-2030: Analysis by Geography
15.4.2.6.1. Oligonucleotide Manufacturing Market in North America, 2020-2030
15.4.2.6.2. Oligonucleotide Manufacturing Market in Europe, 2020-2030
15.4.2.6.3. Oligonucleotide Manufacturing Market in Asia-Pacific and Rest of the World, 2020-2030
- SWOT ANALYSIS
16.1. Chapter Overview
16.2. Comparison of SWOT Factors
- SURVEY ANALYSIS
17.1. Chapter Overview
17.2. Overview of Respondents
17.2.1. Seniority Level of Respondents
17.3. Survey Insights
17.3.1. Type of Offering
17.3.2. Application
17.3.3. Manufacturing Capacity
17.3.4. Location of Manufacturing Facilities
17.3.5. Extent of Outsourcing
17.3.6. Current Market Opportunity
- EXECUTIVE INSIGHTS
18.1. Chapter Overview
18.2. BianoScience
18.2.1. Company Snapshot
18.2.2. Interview Transcript: Tobias Pohlmann, Founder and Managing Director
18.3. IBA Life Sciences
18.3.1. Company Snapshot
18.3.2. Interview Transcript: Joachim Bertram, Chief Scientific Officer and Managing Director
- CONCLUDING REMARKS
19.1. Chapter Overview
19.2. Key Takeaways
- APPENDIX 1: TABULATED DATA
- APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
For additional details, please visit
https://www.rootsanalysis.com/reports/view_document/oligonucleotide-synthesis/304.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
- Pharmaceutical Contract Manufacturing Market (3rd Edition), 2021-2030
- Biopharmaceutical Contract Manufacturing Market (4th Edition), 2021-2030
- Antisense Oligonucleotide Therapeutics Market, 2020-2030
- RNAi Therapeutics Market (2nd Edition), 2019 – 2030
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com
Contact Information
Roots Analysis Private Limited
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091
Facebook - https://www.facebook.com/RootsAnalysis
LinkedIn - https://www.linkedin.com/company/roots-analysis/mycompany/
Twitter - https://twitter.com/RootsAnalysis
Medium - https://medium.com/@RootsAnalysis
Pinterest - https://in.pinterest.com/RootsanalysisPin/_saved/